Our Global Network of Research sites expands from US to Australia. With 1500 sites and growing, we have the ability to source almost all types of biospecimens as well as prospectively collect according to your specific protocol.
iProcess has built one of the world’s most extensive biobanks and sample collection networks, capable of meeting the stringent needs of any disease research program. Whether using specimens for assay development, bench research, or generating big data for your research or development studies, we deliver.

Precise cohorts of data-rich, research-ready specimens to fuel your studies.
Our international network of integrated clinical care centers & specialty laboratories support research and biospecimen collection.

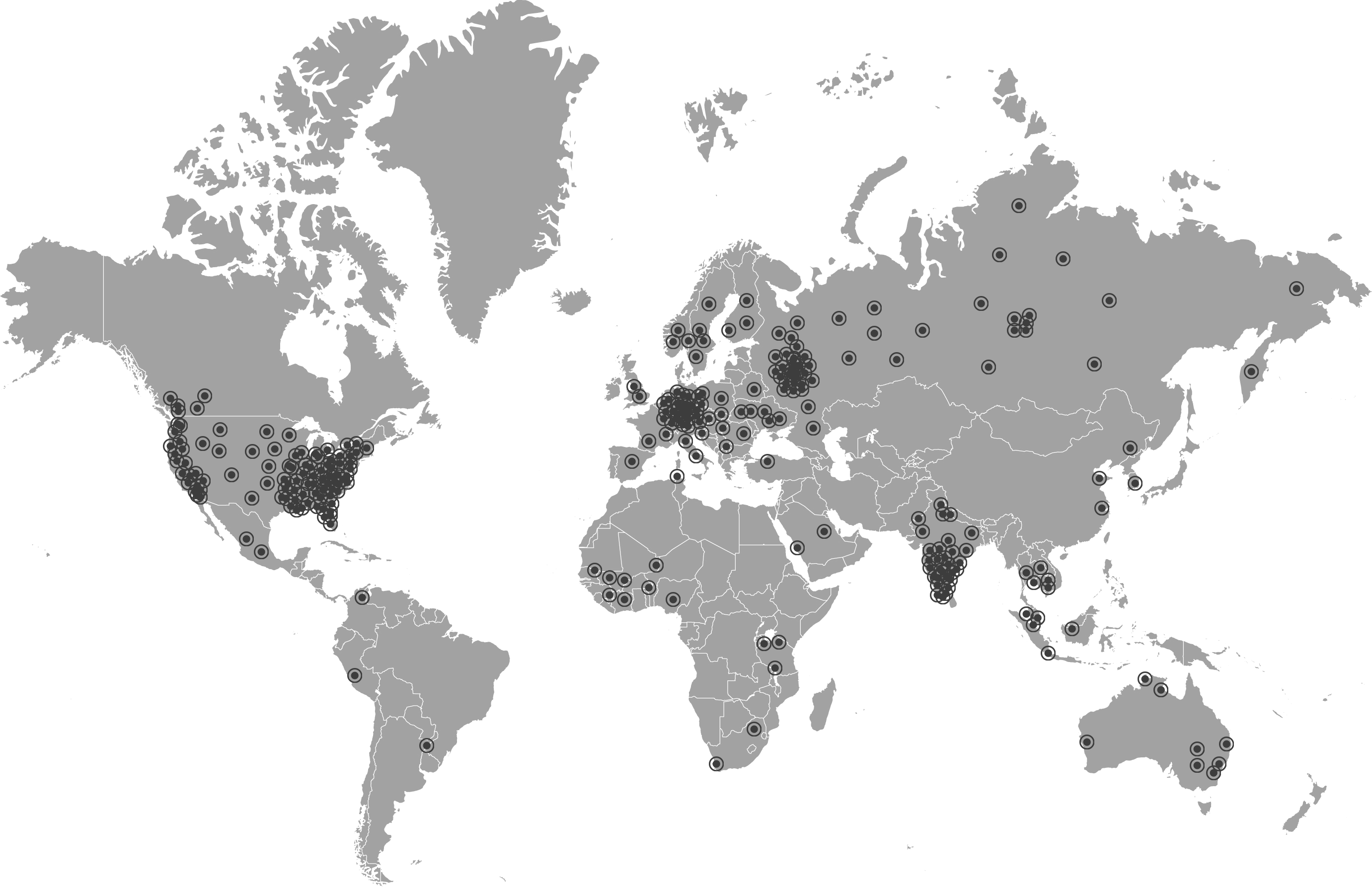
iProcess has thrived and expanded upon its mission and has a network of 1500 sites globally.
iProcess Global Research is your trusted partner for biospecimens. We have over 17 years of experience and maintain a global network of 1500 sites.
Artificial Intelligence and Machine Learning is going to change the way we discover new drugs and diagnose diseases.
iProcess Global Research is your trusted partner for biospecimens. We have over 17 years of experience and maintain a global network of 1500 sites.
iProcess is launching a CLIA Certified laboratory in 2022 to provide testing for our research partners.
Artificial Intelligence and Machine Learning is going to change the way we discover new drugs and diagnose diseases.
iProcess has thrived and expanded upon its mission and has a network of 1500 sites globally.